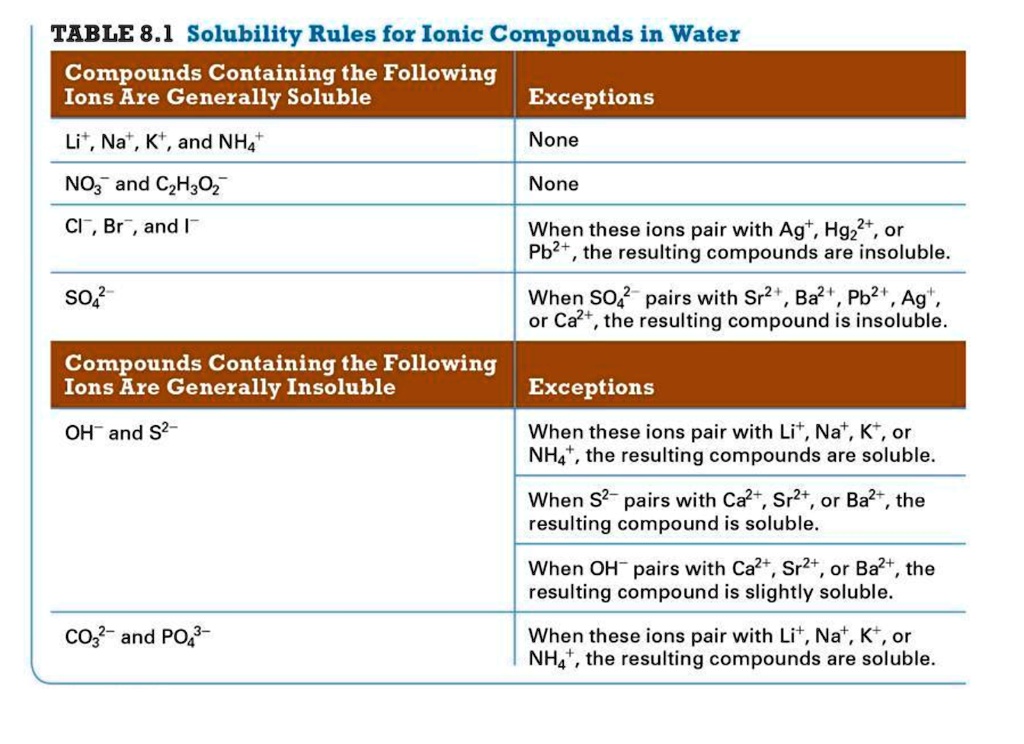
Science Chemistry Chemistry questions and answers Classify these compounds as soluble or insoluble. Solubility rules for ionic compounds in water are shown for your reference. Compound of Rule Li+ , Na+ , K+ , or NH4+ Always soluble NO3− or C2H3O2− Always soluble Cl− , Br− , or This problem has been solved!
Solved You sorted 1 out of 10 items incorrectly. The | Chegg.com
Oct 27, 2022The solubility guidelines in Table \(\PageIndex1\) may be used to predict whether a precipitation reaction will occur when solutions of soluble ionic compounds are mixed together. One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble compound.

Source Image: slideplayer.com
Download Image
Classify these compounds as soluble or insoluble. Skip to main content. General Chemistry Start typing, then use the up and down arrows to select an option from the list. … Classify these compounds as soluble or insoluble. Verified Solution. This video solution was recommended by our tutors as helpful for the problem above. 2m. Play a video: 85.
Source Image: numerade.com
Download Image
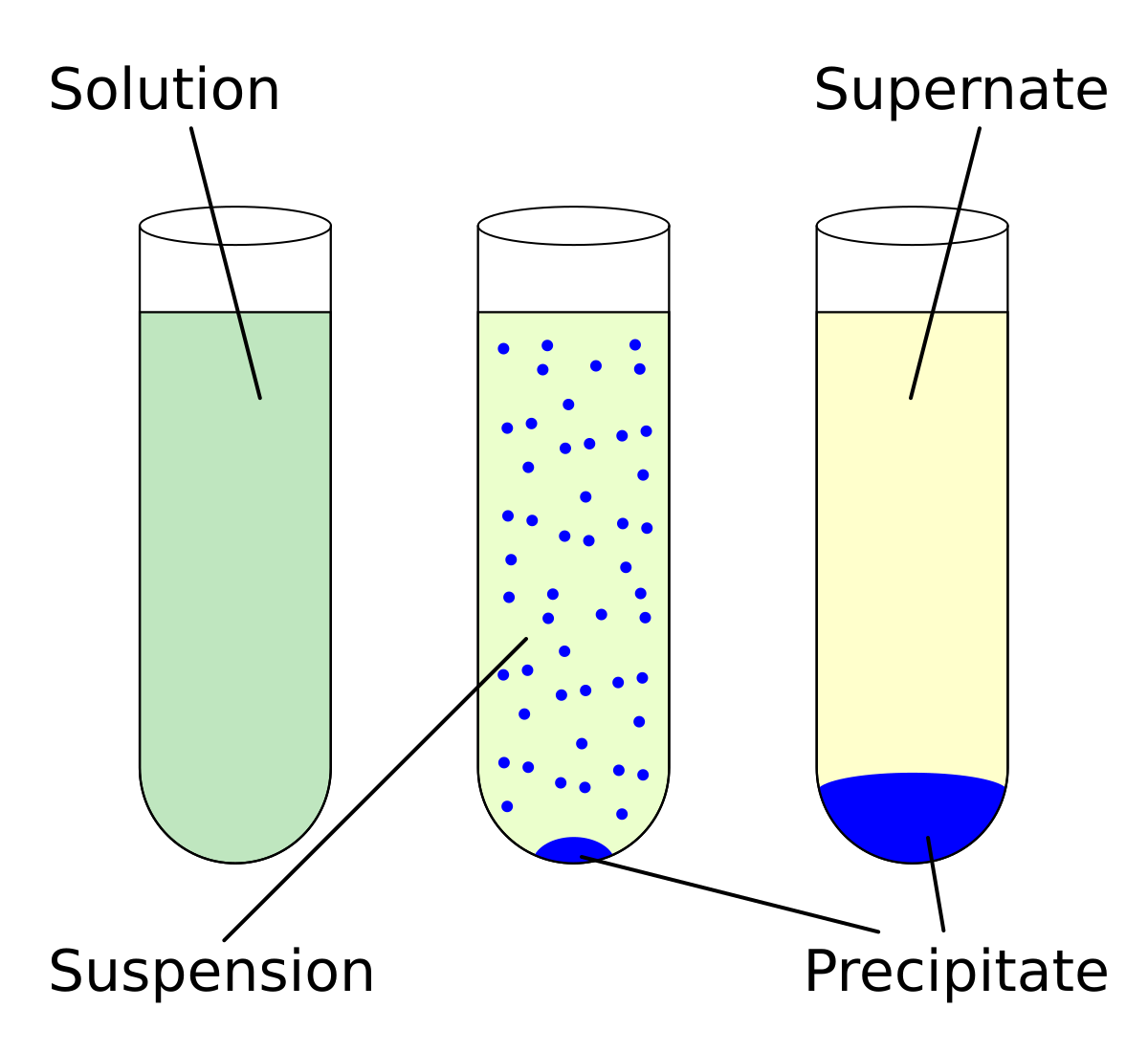
Solved classify these compounds as soluble or insoluble. | Chegg.com Jan 30, 20232AgNO3 + Na2S → Ag2S + 2NaNO3 (1) (1) 2 A g N O 3 + N a 2 S → A g 2 S + 2 N a N O 3. The products of the reaction must be examined; if either of the substances formed in the reaction is insoluble, a precipitate will form. Considering NaNO 3, Rule #3 states that nitrates tend to be soluble. A precipitate of this compound will not form.
Source Image: en.wikipedia.org
Download Image
Classify These Compounds As Soluble Or Insoluble
Jan 30, 20232AgNO3 + Na2S → Ag2S + 2NaNO3 (1) (1) 2 A g N O 3 + N a 2 S → A g 2 S + 2 N a N O 3. The products of the reaction must be examined; if either of the substances formed in the reaction is insoluble, a precipitate will form. Considering NaNO 3, Rule #3 states that nitrates tend to be soluble. A precipitate of this compound will not form. Science > UP Class 6th Science > Substances and groups of substances > Classification of objects on the basis of various characteristics Soluble and Insoluble | Some materials are soluble in water, while some aren’t. Materials can be categorised according to that property. Created by Vibhor Pandey. Questions Tips & Thanks Sort by: Top Voted
Solubility – Wikipedia
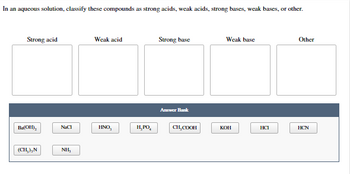
Classify each of these soluble solutes as a strong electrolyte, a weak electrolyte, or a nonelectrolyte. Sulfuric acid – H2SO4 Lithium hydroxide- LiOH Carbonic acid- H2CO3 Ethyl amine- CH3CH2NH2 Sodium bromide- NaBr Butanol- C4H9OH Sucrose- C12H22O11 Click the card to flip 👆 PART A: Strong Electrolyte (completely ionizes to produce ions): BD, PR Answered: In an aqueous solution, classify these… | bartleby
Source Image: bartleby.com
Download Image
Question #c22fc + Example Classify each of these soluble solutes as a strong electrolyte, a weak electrolyte, or a nonelectrolyte. Sulfuric acid – H2SO4 Lithium hydroxide- LiOH Carbonic acid- H2CO3 Ethyl amine- CH3CH2NH2 Sodium bromide- NaBr Butanol- C4H9OH Sucrose- C12H22O11 Click the card to flip 👆 PART A: Strong Electrolyte (completely ionizes to produce ions): BD, PR
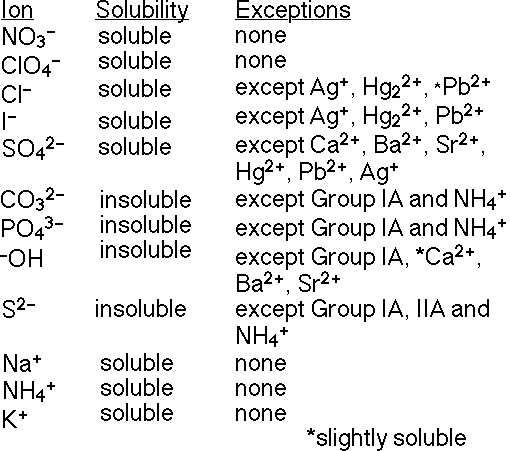
Source Image: socratic.org
Download Image
Solved You sorted 1 out of 10 items incorrectly. The | Chegg.com Science Chemistry Chemistry questions and answers Classify these compounds as soluble or insoluble. Solubility rules for ionic compounds in water are shown for your reference. Compound of Rule Li+ , Na+ , K+ , or NH4+ Always soluble NO3− or C2H3O2− Always soluble Cl− , Br− , or This problem has been solved!
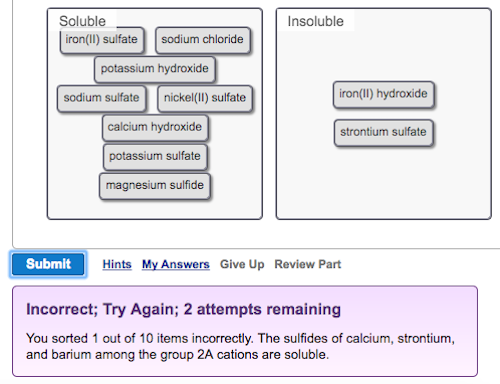
Source Image: chegg.com
Download Image
Solved classify these compounds as soluble or insoluble. | Chegg.com Classify these compounds as soluble or insoluble. Skip to main content. General Chemistry Start typing, then use the up and down arrows to select an option from the list. … Classify these compounds as soluble or insoluble. Verified Solution. This video solution was recommended by our tutors as helpful for the problem above. 2m. Play a video: 85.
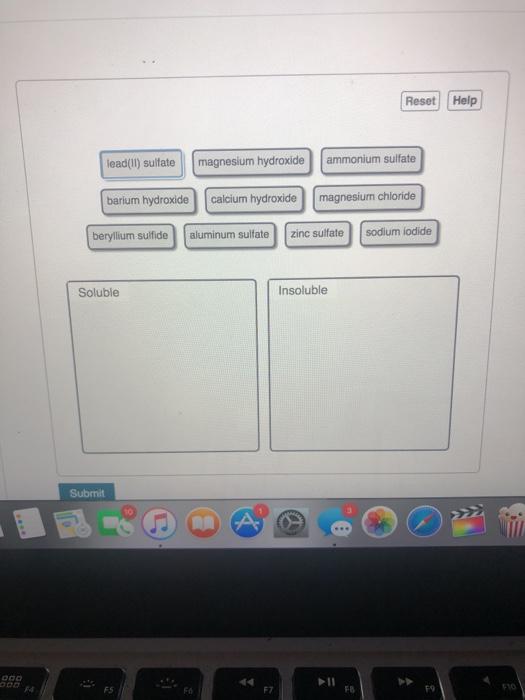
Source Image: chegg.com
Download Image
IJMS | Free Full-Text | An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS) Classify each of the following compounds as soluble or insoluble in water: \\ (a) \mathrm PbCl_2, PbCl2, (b) \mathrm (NH_4)_3PO_4, (NH4)3PO4, (c) \mathrm Fe (OH)_3. Fe(OH)3. Solutions Verified Solution A Solution B Answered 1 year ago Create an account to view solutions Continue with Google Sign up with email Recommended textbook solutions
Source Image: mdpi.com
Download Image
SOLVED: The accompanying diagram represents an aqueous solution of either MgCl2, KCl, or K2SO4. Which solution does the drawing best represent? Classify these ionic compounds as soluble or insoluble in water: (a) Jan 30, 20232AgNO3 + Na2S → Ag2S + 2NaNO3 (1) (1) 2 A g N O 3 + N a 2 S → A g 2 S + 2 N a N O 3. The products of the reaction must be examined; if either of the substances formed in the reaction is insoluble, a precipitate will form. Considering NaNO 3, Rule #3 states that nitrates tend to be soluble. A precipitate of this compound will not form.
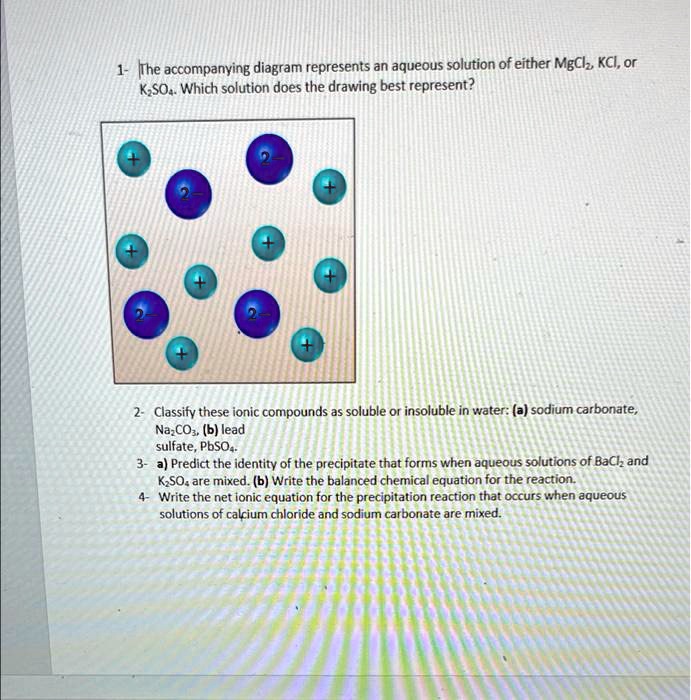
Source Image: numerade.com
Download Image
Use the molecular representations shown here to classify each com… | Channels for Pearson+ Science > UP Class 6th Science > Substances and groups of substances > Classification of objects on the basis of various characteristics Soluble and Insoluble | Some materials are soluble in water, while some aren’t. Materials can be categorised according to that property. Created by Vibhor Pandey. Questions Tips & Thanks Sort by: Top Voted

Source Image: pearson.com
Download Image
Question #c22fc + Example
Use the molecular representations shown here to classify each com… | Channels for Pearson+ Oct 27, 2022The solubility guidelines in Table \(\PageIndex1\) may be used to predict whether a precipitation reaction will occur when solutions of soluble ionic compounds are mixed together. One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble compound.
Solved classify these compounds as soluble or insoluble. | Chegg.com SOLVED: The accompanying diagram represents an aqueous solution of either MgCl2, KCl, or K2SO4. Which solution does the drawing best represent? Classify these ionic compounds as soluble or insoluble in water: (a) Classify each of the following compounds as soluble or insoluble in water: \\ (a) \mathrm PbCl_2, PbCl2, (b) \mathrm (NH_4)_3PO_4, (NH4)3PO4, (c) \mathrm Fe (OH)_3. Fe(OH)3. Solutions Verified Solution A Solution B Answered 1 year ago Create an account to view solutions Continue with Google Sign up with email Recommended textbook solutions