Experiment 3: Osmosis – Direction and Concentration Gradients In this experiment, we will investigate the effect of solute concentration on osmosis. A semi-permeable membrane (dialysis tubing) and sucrose will create an osmotic environment similar to that of a cell.
Experiment 3 Osmosis – Direction and Concentration Gradients.docx – Experiment 3: Osmosis – Direction and Concentration Gradients In this experiment | Course Hero
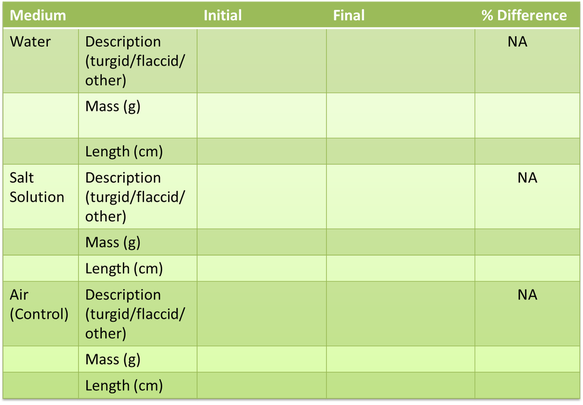
EXPERIMENT 3: OSMOSIS – DIRECTION AND CONCENTRATION GRADIENTS. Hypothesis: If the solutions are hypertonic then the net displacement will be greater. Scientific Reasoning: A hypertonic solution would cause water to flow outside the cell. Data Tables. Table 6: Sucrose Concentration vs. Tubing Permeability. Band Color Sucrose % Initial Volume (mL)
Source Image: slideserve.com
Download Image
Experiment 3: Osmosis – Direction and Concentration GradientsIn this experiment, we will investigate the effect of solute concentration on osmosis. A semi-permeable membrane (dialysis tubing) and sucrose will create an osmotic environment similar to that of a cell.
Source Image: byjus.com
Download Image
Development and long-term field test of electrodeionization for decentralized desalination facility – ScienceDirect
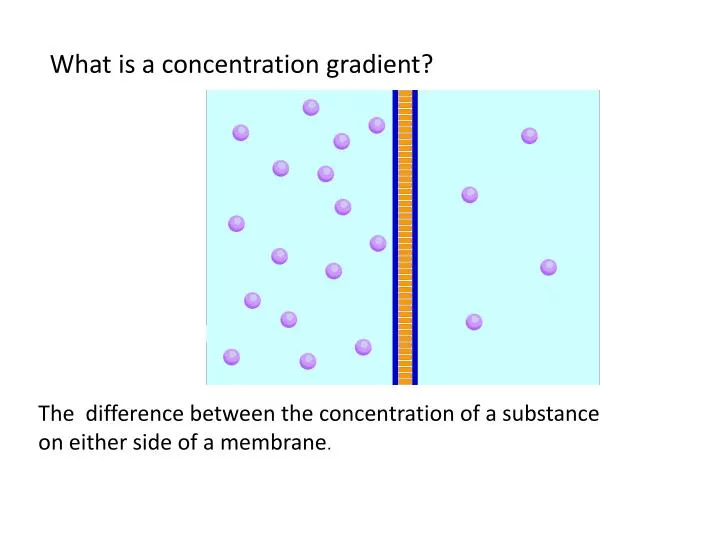
AboutTranscript. Diffusion refers to the movement of molecules from an area of high concentration to an area of lower concentration. Osmosis is a type of diffusion specifically for water molecules moving across a semi-permeable membrane. A concentration gradient is the difference in concentration of a substance between two areas, which drives

Source Image: yumpu.com
Download Image
Experiment 3 Osmosis Direction And Concentration Gradients
AboutTranscript. Diffusion refers to the movement of molecules from an area of high concentration to an area of lower concentration. Osmosis is a type of diffusion specifically for water molecules moving across a semi-permeable membrane. A concentration gradient is the difference in concentration of a substance between two areas, which drives
2 ABSTRACT. The purpose of this experiment is to establish a correlation between the concentration gradient and the rate of osmosis. Different variables were put into consideration, mass, concentration gradient, rate of osmosis, and time and combining them made it possible to come up with relevant findings that assisted in understanding the experiment.
Diffusion and Osmosis
EXPERIMENT 3: OSMOSIS – DIRECTION AND CONCENTRATION GRADIENTS Hypothesis. Scientific Reasoning Attach TWO images showing initial experiment setup and final results Data Tables Table 6: Sucrose Concentration vs. Tubing Permeability Band Color Sucrose % Initial Volume (mL) Final Volume (mL) Net Displacement (mL) Yellow 30% 10mL 14mL 4mL Red 15% 10mL 12mL 2mL Blue 3% 10mL 9mL – 1ml Green 3% 10mL
The movement of sucrose in plants can be modelled using laboratory appara..
Source Image: askfilo.com
Download Image
Osmosis, diffusion, active transport | PPT
EXPERIMENT 3: OSMOSIS – DIRECTION AND CONCENTRATION GRADIENTS Hypothesis. Scientific Reasoning Attach TWO images showing initial experiment setup and final results Data Tables Table 6: Sucrose Concentration vs. Tubing Permeability Band Color Sucrose % Initial Volume (mL) Final Volume (mL) Net Displacement (mL) Yellow 30% 10mL 14mL 4mL Red 15% 10mL 12mL 2mL Blue 3% 10mL 9mL – 1ml Green 3% 10mL
Source Image: slideshare.net
Download Image
Experiment 3 Osmosis – Direction and Concentration Gradients.docx – Experiment 3: Osmosis – Direction and Concentration Gradients In this experiment | Course Hero
Experiment 3: Osmosis – Direction and Concentration GradientsIn this experiment, we will investigate the effect of solute concentration on osmosis. A semi-permeable membrane (dialysis tubing) and sucrose will create an osmotic environment similar to that of a cell.

Source Image: coursehero.com
Download Image
Development and long-term field test of electrodeionization for decentralized desalination facility – ScienceDirect
Experiment 3: Osmosis – Direction and Concentration Gradients In this experiment, we will investigate the effect of solute concentration on osmosis. A semi-permeable membrane (dialysis tubing) and sucrose will create an osmotic environment similar to that of a cell.

Source Image: sciencedirect.com
Download Image
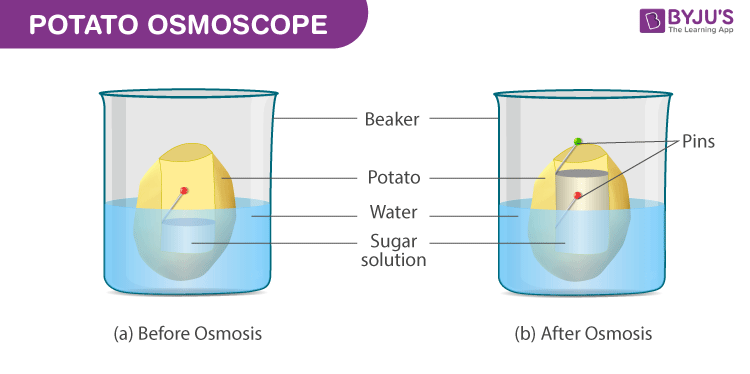
Investigating osmosis in potatoes – Biology : Explanation & Exercises – evulpo
AboutTranscript. A concentration gradient occurs when the concentration of particles is higher in one area than another. In passive transport, particles will diffuse down a concentration gradient, from areas of higher concentration to areas of lower concentration, until they are evenly spaced. Created by Sal Khan.
Source Image: evulpo.com
Download Image
Investigate Osmosis using Potato Strips – Brilliant Biology Student
AboutTranscript. Diffusion refers to the movement of molecules from an area of high concentration to an area of lower concentration. Osmosis is a type of diffusion specifically for water molecules moving across a semi-permeable membrane. A concentration gradient is the difference in concentration of a substance between two areas, which drives
Source Image: brilliantbiologystudent.weebly.com
Download Image
Experiment 3 Osmosis – Direction and Concentration Gradients.docx – Experiment 3: Osmosis – Direction and Concentration Gradients In this experiment | Course Hero
2 ABSTRACT. The purpose of this experiment is to establish a correlation between the concentration gradient and the rate of osmosis. Different variables were put into consideration, mass, concentration gradient, rate of osmosis, and time and combining them made it possible to come up with relevant findings that assisted in understanding the experiment.

Source Image: coursehero.com
Download Image
Osmosis, diffusion, active transport | PPT
Experiment 3 Osmosis – Direction and Concentration Gradients.docx – Experiment 3: Osmosis – Direction and Concentration Gradients In this experiment | Course Hero
EXPERIMENT 3: OSMOSIS – DIRECTION AND CONCENTRATION GRADIENTS. Hypothesis: If the solutions are hypertonic then the net displacement will be greater. Scientific Reasoning: A hypertonic solution would cause water to flow outside the cell. Data Tables. Table 6: Sucrose Concentration vs. Tubing Permeability. Band Color Sucrose % Initial Volume (mL)
Development and long-term field test of electrodeionization for decentralized desalination facility – ScienceDirect Investigate Osmosis using Potato Strips – Brilliant Biology Student
AboutTranscript. A concentration gradient occurs when the concentration of particles is higher in one area than another. In passive transport, particles will diffuse down a concentration gradient, from areas of higher concentration to areas of lower concentration, until they are evenly spaced. Created by Sal Khan.